Background: The treatment goals of Polycythemia Vera (PV) have been focused on reducing the thrombotic and hemorrhagic risks and preventing disease transformation. A short-term way to confirm the effect of treatment is to check peripheral blood count remission. Recently, published researches have demonstrated that JAK2 driver mutation is associated with not only disease progression but hematologic response. Although it is still controversial whether a decrease in JAK2 mutation should be one of the treatment goals or not, the molecular response (MR) is frequently assessed in clinical trials. Moreover, the interest in treatment is moving toward disease modification. Ropeginterferon alfa-2b(P1101) showed it was more effective in achieving durable hematological and molecular remissions than hydroxyurea and well tolerated during long-term application. However, there are limited data on the clinical relevance of MR during P1101 and the efficacy and safety of P1101 in Eastern Asians.
Aims: The aim of this study is to evaluate clinical and molecular response, and the association between efficacy and MR. In addition, the safety and tolerability of P1101 were also collected.
Methods: This single-arm, open-label study has been performed in 16 hospitals in Korea. Patients were eligible if 19 years or older with PV diagnosed by WHO's 2016 criteria, requiring cytoreductive therapy and elevated hematocrit (Hct) (>45%). Patients were treated with P1101, S.C. q2w, at a starting dose of 250 µg, followed by 350 µg(week 2), 500 µg(week 4), and thereafter until week 48 (core study period). The JAK2 Val617Phe allele burden was assessed every 3 months. The chi-square test was used to determine any association between complete hematologic response (CHR) and MR using the Phi coefficient as a measure of strength association.
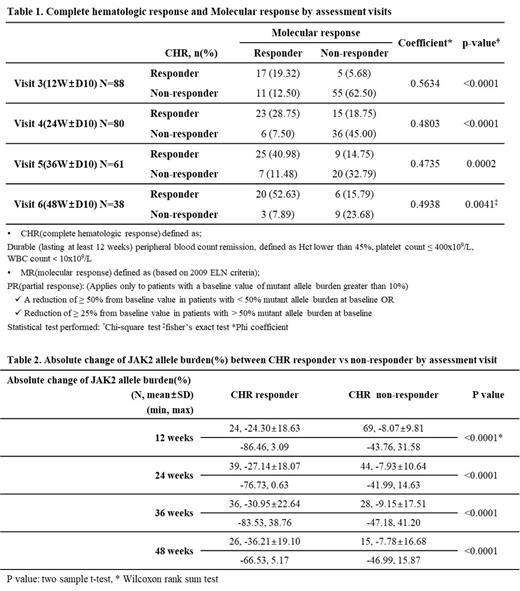
Results: With a data cut-off date of 10 Jul 2023, a total of 99 patients were enrolled and 77 patients are under treatment including extension period in the study. Twenty patients dropped out including 2 patients due to adverse events and 2 patients completed core study. Total 95 patients were included in this analysis as FA (full analysis) set: 52 (54.7%) patients was HU-naïve and 43 (45.3%) patients was HU-resistant/intolerant(R/I). The median age was 58 years (range, 26-81) and 53.7% were male. The percentagesof patients with low and high risk were 56.8% and 43.2%, repectively. Until now, 94, 87, 79, and 60 patients were evaluable at 3, 6, 9, and 12 months, respectively. CHR was achieved in 25 (27%) of 94, 40 (46%) of 87 patients, 45 (57%) of 79 patients, and 38 (63%) of 60 patients at 3, 6, 9, and 12 months, respectively. Twenty-eight (32%) of 88, 29 (36%) of 80 patients, 32 (52%) of 62 patients, and 23 (61%) of 38 patients achieved the MR at 3, 6, 9, and 12 months, respectively. The Phi Coefficient for determining of association between CHR and MR was 0.56 (P<0.0001), 0.48 (P<0.0001), 0.4735 (P=0.0002), and 0.4938 (P=0.0041) at each time point (Table 1). Regarding the calculated absolute change from baseline of JAK2 allele burden at each time point, a faster and deeper decreasing pattern was shown in CHR responders compared to non-responders (Table 2). Regarding the Hct, platelets, and WBC, the mean value of all parameters significantly decreased to the normal range after 6 months and were consistent in normal until 12 months. Moreover, there is also no significance difference between HU naïve vs R/I and low vs High risk. In terms of safety, a total of 160 treatment-emergent adverse events (TEAEs) and 84 treatment-related adverse events (TRAE) were reported. The most common TEAEs (%, n/95) were alopecia (18%, 17/95) and adverse events related to liver function (16%, 15/95), respectively. Onset time of TRAEs distributed 8, 22, 27, 18, and 8 during week 0-4, 4-12, 12-24, 24-36, and 36-48, respectively. There were no unexpected TEAEs. Two serious adverse drug reactions (1 hepatotoxicity and 1 bipolar disorder) were reported.
Conclusion: The updated data showed consistent results with the previous 2022 ASH meeting abstract that ropeginterferon alfa-2b therapy, with rapid dose optimization, induced hematological response, reduced JAK2 allele burden, and was well tolerated in Korean patients. Moreover, we clarified the association between efficacy and MR as assessed by reduction in JAK2 allele burden. More data will be updated during the meeting.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal